Iodine Overview
I will risk losing a few followers with this post.
"It ain't what you don't know that gets you into trouble. It's what you know for sure that just ain't so." - Mark Twain
Iodine is one of the most hated and most loved nutrients depending who you ask. Even my favorite nutrition group, the Ray Peat-arians, are typically against iodine supplementation, and for good reason when first examining the facts. I certainly don't want to say they're "wrong" because I really don't know. I can only tell you what I feel from it, and try to come up with reasons why. And I definitely don't want to throw stones at the physiological giant Ray Peat. But, we need to remember that Ray Peat does recommend consuming foods with iodine such as shellfish, milk, cheese and potatoes. I'm not recommending mega-dosing iodine because I believe in balance, but I am advocating paying attention to iodine consumption versus other halogen consumption.
I used iodine heavily prior to finding the work of Ray Peat. This was around the time I was regularly sucking down blended chia seeds, hemp seeds, pumpkin seeds and the worst tasting of all, sesame seeds. Hey, they've got lots of calcium. I still had good results from iodine at that time. I sometimes think that my initial outstanding results with Peatarianism (see my Eat as Much as Possible post) were at least partially from the iodine I had built up in my system.
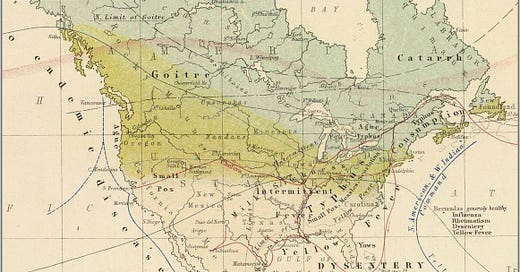
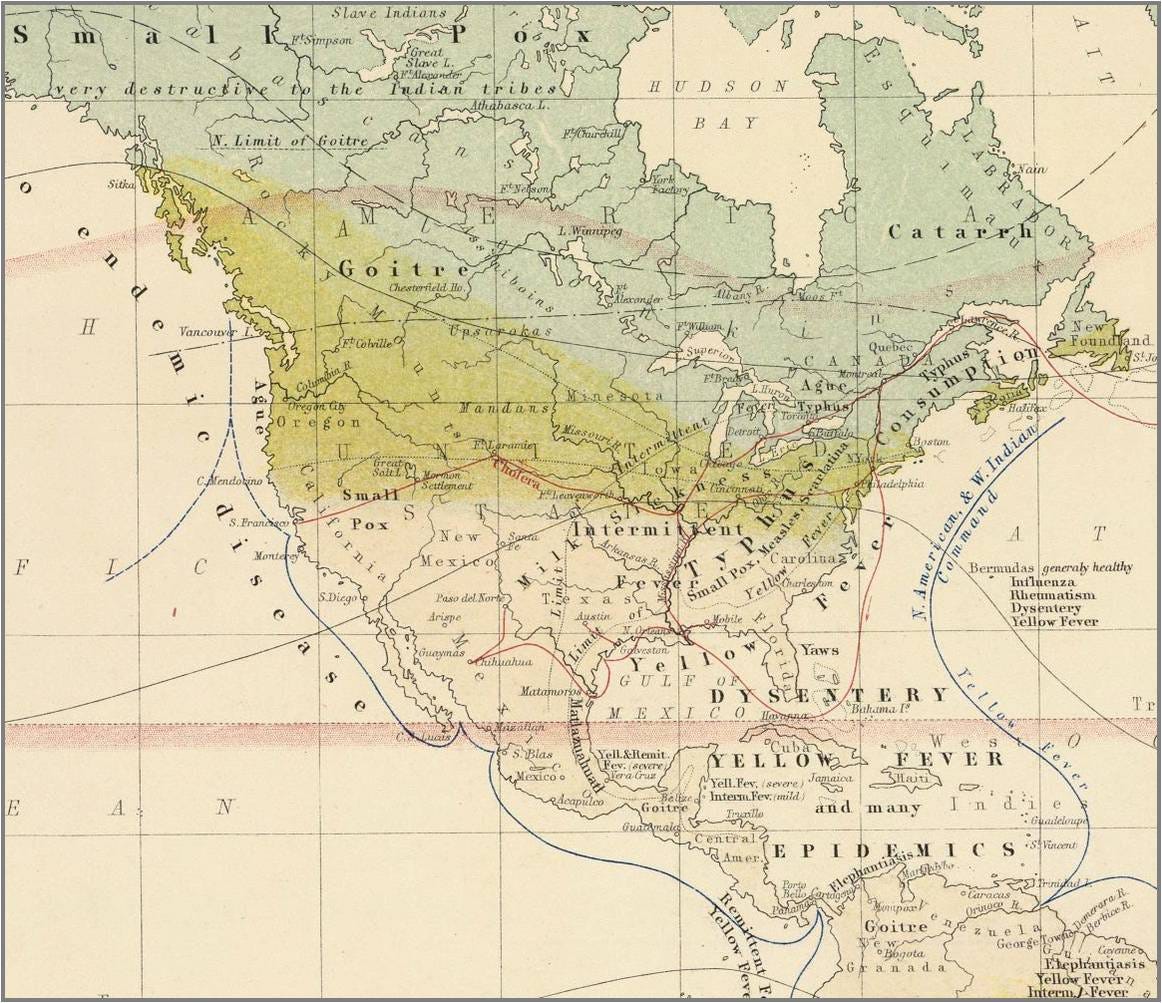
The RDA for iodine is 150 micrograms. That means .000150 grams. A tiny amount, right - so why would we need to take more? Typically we can get this much from potatoes, eggs and milk, depending on the source of aforementioned foods. Animals grazing near or in the goiter (goitre) zones are likely to have less iodine in their products than animals that graze elsewhere. See the goiter zones in Alexander Keith Johnston’s 1856 map of North American disease patterns. This is likely a result of the climate, erosion from glaciers and the topographic features of the northern part of the US and Canada. Glaciers likely melted and took a lot of the iodine (and other trace minerals) to the Gulf of Mexico via the Missouri and Mississippi river systems.
Turns out that the RDA was really only calculated to be the bare minimum necessary for preventing goiter. In theory, some goiters occur because the thyroid tissue expands to absorb additional iodine. Of course, that is not enough of a justification for you to begin guzzling the gross metallic tasting brown stuff. I aim to provide some real research and math to justify considering iodine supplementation. As always, you're the master of your own ship so you need to find what works for you.
I weigh about 200 lbs or 90 kgs. Based on numbers I've found on the internet, the body's daily requirement is 1.7 mcg / kg of thyroid hormone T4, and .5 mcg / kg of thyroid hormone T3. This means I need about 153 mcg T4 and 46 mcg T3 (30% of the T4 number) on a daily basis. These numbers should also be used for accurate thyroid dosing to ensure you're getting enough thyroid for your body. I will post more about my theories on this later.
A T4 molecule contains four atoms of iodine, or about 65.34% of the mass. This is 153 mcg * 65.34% = 99.9 mcg of iodine (1)
A T3 molecule contains three atoms of iodine, or about 59.73% of the mass. This is 46 mcg * 59.73% = 27.4 mcg of iodine (2)
This is a total of 127.4 mcg of iodine needed for production of thyroid hormone only. This actually lines up quite well with the RDA value of 150 mcg to prevent goiter. However, the problem, as always, gets more complex.
Why People May Need More than the RDA of 150 micrograms
Environmental toxins
The halogen elements fluoride and bromide are abundant in our "modern" environment. These fill iodine receptors and displace iodine in the body. "Fluoride, the negative ion of the element fluorine, easily displaces iodine in the body because it is much lighter and therefore more reactive. In fact the activity of any one of the halogens is inversely proportion to its atomic weight (14). Iodine has the highest atomic mass and is therefore more easily displaced by the smaller halogens. Here are examples of where you come in contact with them, and typically how much.
Bromide - In Food, Pesticides, Laundry Detergent, Furniture
An American typically consumes or absorbs 2 to 8mg on a daily basis (3) - which is 13 to 53 times more than the RDA (150 mcg) for iodine. Bromide is found in pesticides (methyl bromide), bread products (potassium bromate in "brominated flour"), and brominated vegetable oil that may be added to citrus-flavored drinks like Mountain Dew or Gatorade, hot tub cleansers, prescription drugs, plastics and some fabric dyes (4).
In thyroid cancer patients, Malenchenk found bromide levels are 50 times higher than normal thyroid tissue (5). Rats fed the minimal amount of bromine expected from the environment for 16 and 66 days underwent goiter-like changes (6), a potential case of bromide dominance. Rats exposed to the brominated flame retardant compound (in furniture), bromocyclodecane, showed consistent effects on the thyroid hormone axis, including decreased T4. Thyroid gland cells increased in size and had larger nuclei, indicating increased synthetic bromide activity. (7) With more than environmentally expected intake of bromide, fully one-third of the iodine content in the thyroids of rats was replaced by bromide. (8,9)
Beyond the thyroid, bromide has detrimental effects on the brain. Bromide toxicity has been implicated in mental conditions such as transitory schizophrenia (10). Additionally, "Psychiatric symptoms (of bromism) may include, in the earlier stages, disinhibition, self-neglect, fatigue, sluggishness, impairment of memory and concentration, irritability or emotional instability, and depression" (11). Often, psychiatric drugs are prescribed to deal with these symptoms which may actually worsen bromism. Some contain bromide (Celexa) and all SSRIs contain fluoride (Paxil, Prozac, Luvox, Lexapro) except Zoloft.
Prevously, bromide (Neurosine pharmaceutical) was used to suppress women's sex drive in the 1950s (12).
The UK banned bromate in bread in 1990. Canada banned bromate in bread in 1994. The U.S. has not banned bromate in spite of a petition submitted in 1999 by the Center for Science in the Public Interest. The center claimed that the FDA has known for years that bromate causes cancer in lab animals (13)
You can best reduce your chance of getting bromism by avoiding bromide/bromate/bromine. Otherwise, ensuring your iodine levels are sufficient (on par with bromide consumption) may be a decent defense from this environmental toxin crowding out iodine in your thyroid and other tissues.
Fluoride - In Water, In Food: Time to Get out the Tinfoil hat, right? Well, let's look at the facts.
"Fluoride is a universal G-protein activator/inhibitor. The stimulation of certain G-proteins occurs due to the toxic effects of fluoride, which has the effects of switching off the uptake into the cell of the active thyroid hormone (14)." TSH is commonly used to estimate the activity of the thyroid. A high TSH means the thyroid isn't adequately responding to the TSH so the pituitary gland "tries" harder. "The TSH output from pituitary gland is inhibited by fluoride, thus reducing thyroid output from thyroid glands. Fluoride competes for the receptor sites on the thyroid gland which respond to TSH; so that less of this hormone reaches the thyroid gland and fewer hormones are manufactured (Wilson and DeEds 1940; Susheela et al. 2005)." (14)
A study in India was conducted on 60 children examining fluoride intake's effects. They were broken into subgroups - those that have dental fluorosis and those that don't - subgroups 1A and 1B. Group 1A where 15 children were selected in India with fluoride levels in water up to 2.6 mg / L, and Group 1B where 15 children had water levels were up to 5.1 mg / L. The group 2 (ten) children were of similar socio-economic status, but had "safe" (<1mg / L) levels of fluoride in their water. Group 1 had 43 children of 60 (71%) with derangement of thyroid hormones - FT3, FT4 and TSH. Group 2 had 1 child of 10 with derangement (14).
The US government previously recommended that the fluoridated water supply to contained .7 mg / L to 1.2 mg / L. Now, they are recommending no more than .7 mg / L after about 41% of adolescents are suffering from fluorosis - a condition affecting the appearance of tooth enamel (8). Most "health" experts recommend consuming 8 cups of water in a day. This is approximately two liters, and therefore 2 mg of fluoride. Which, again, is 13 times more fluoride than the RDA of iodide.
Indeed, data has shown that fluoride can also affect fertility and sex drive similar to bromide. "The annual total fertility rate (TFR) for women in the age range 10-49 yr was calculated for the period 1970-1988. For each region separately, the annual TFR was regressed on the fluoride measure and sociodemographic covariables. Most regions showed an association of decreasing TFR with increasing fluoride levels. Meta-analysis of the region-specific results confirmed that the combined result was a negative TFR/fluoride association with a consensus combined p value of .0002-.0004, depending on the analytical scenario" (17).
Black tea is also a fairly significant source (<2 mg / L) of fluoride. Higher quality black teas typically have less fluoride. Green and white teas also typically have less fluoride than black tea. (9) Toothpaste is another potentially significant source of fluoride intake. Many commercial baked goods are made with tap-water. Same with a lot of sports drinks. When making baked goods such as bread, hamburger buns or cupcakes, flour is made with water. When the temperature rises, water becomes steam and leaves behind the minerals fluoride and bromide in a very digestible form without excess liquid.
In one study, rats with insufficient iodine status were fed fluoride at 10 mg a day. They gained weight and their thyroids gained weight (18).
Again, either avoid fluoride by using steam distilled water / spring water in everything you do, and managing tea intake, or consider defending yourself by establishing iodine sufficiency status on par with your expected consumption of fluoride.
Chlorate, Perchlorate - In Water, In Fertilizer and Food Prepared with Tap Water
"Perchlorate inhibits thyroidal iodine uptake and subsequently decreases thyroid hormone production." (25). The Health Reference Level for chlorate in tap water is 210 mcg / L. This isn't very much, but it's still greater than the RDA of iodine. In addition, 35% of locations sampled in the US had levels higher than that, including a few with values in the 700 mcg / L to 1000 mcg / L (26).
Along the Mississippi river valley, sodium chlorate is heavily used as a fertilizer for soybeans, corn, cotton and rice. Most of Mississippi, Iowa and the northern part of Louisiana use greater than 2.57 lbs of this fertilizer per acre (26). This will accumulate in the water supply everywhere downstream of this use.
Even the EPA identifies that "Chlorate, similar to perchlorate, is a goitrogen and can decrease iodide uptake through competitive inhibition leading to the formation of goiters (enlargement of the thyroid). The USEPA cited the interference of perchlorate with iodine transport into the thyroid gland and low iodine uptake in general as the major health concern requiring a perchlorate MCL (USEPA 2011b), and thus might be 22 another factor EPA considers in regulating chlorate." (26)
Polyunsaturated fats - In Food, In Everything
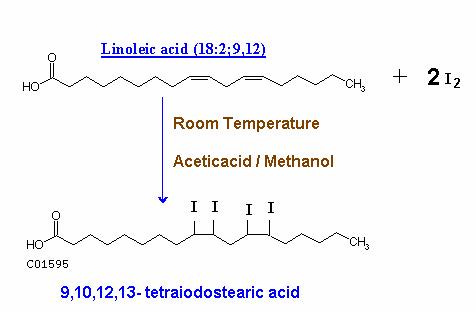
Using iodine is one of the methods for determining how unsaturated an oil is. The number of grams of iodine absorbed by 100 grams of a given oil is called the iodine value. You can read about it more on Wikipedia here. The more unsaturated the more iodine it absorbs - some very unsaturated oils actually absorb more grams of iodine than their own mass.
Unsaturated fatty acids are the those containing carbon-carbon double bonds. Iodine atoms react across the carbon-carbon bonds. The iodine will attach itself over a double bond to make a single bond where an iodine atom is now attached to each carbon atom. Higher iodine numbers do not refer to the amount of iodine in the oil, but rather the amount of iodine needed to "saturate" the oil, or break all the double bonds (23). Therefore, the more unsaturated, the more iodine it takes to break all the double bonds.
Most Peat-arians know the value of Saturated oils - those without double-bonds that resist becoming peroxides or hydrogenated at high temperatures. They are also are well-versed in the dangers associated with high polyunsaturated fat intake due to their effects on the thyroid gland, inflammation, and blood sugar metabolism. Every gram of our favorite soybean oil absorbs between 1.2 and 1.36 grams of iodine. Yes, you read that right. 1.36 GRAMS! (19)
Linoleic Acid Becomes Similar to Stearic Acid